
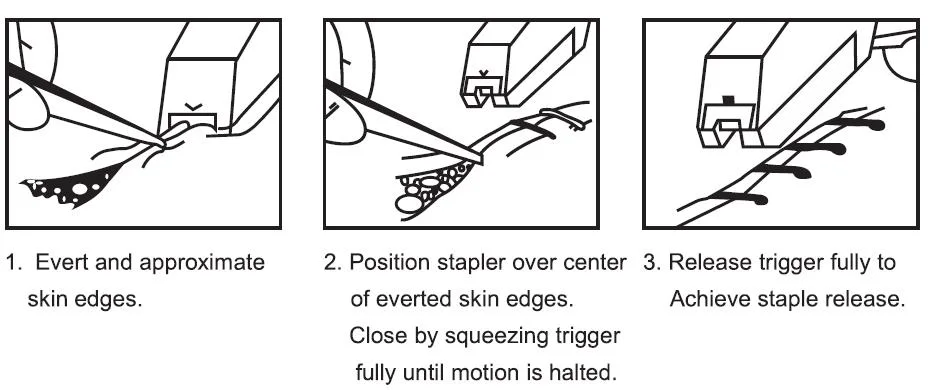
It is applicated in Abdominal, Gynecological, Orthopedic, and Thoracic surgery for skin closure.

Specification
| Product Code | Staple Size | Staple Quantity | Remover Code | Skin interior tissue |
| CSPF-15W | 7.4×4.6 mm | 15 pcs | CSPF-CW | More than 5 mm Conventional Use |
| CSPF-25W | 7.4×4.6 mm | 25 pcs | CSPF-CW | |
| CSPF-35W | 7.4×4.6 mm | 35 pcs | CSPF-CW | |
| CSPF-15R | 6.0×4.0 mm | 15 pcs | CSPF-CW | Less than 5 mm Ankle, Knee, Head |
| CSPF-25R | 6.0×4.0 mm | 25 pcs | CSPF-CW | |
| CSPF-35R | 6.0×4.0 mm | 35 pcs | CSPF-CW |
Using Instruction
Hospital Departments
General surgical operation, Sardiothoracic surgery, Plastic surgery, Neurosurgery operation, Gynecologic surgery, Burn surgery, Emergency operation and Field rescue.
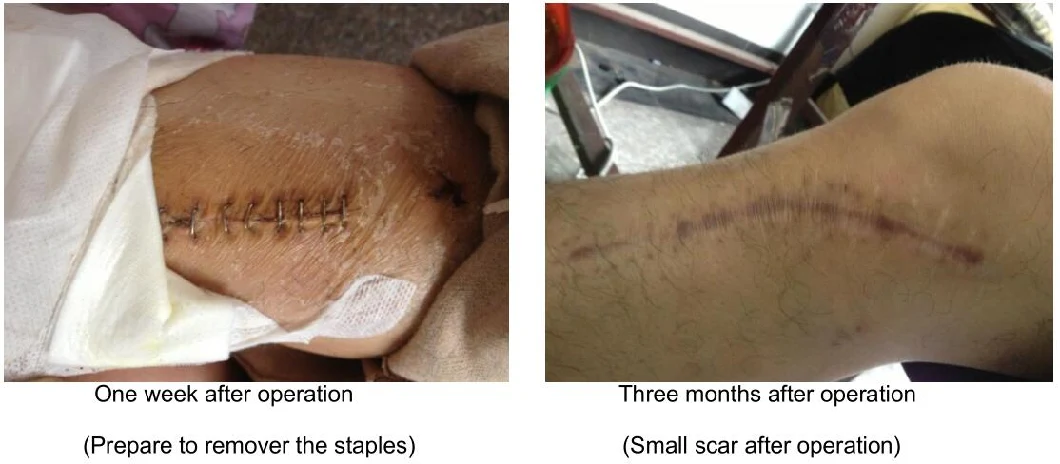
Postoperative Effect
Features:
Small stitched wound, avoid injury to epidermal tissue again and well for wound healing.
Smooth stitched wound and small scar after operation.
Shorten time for operation and decrease the pain for patients.
Reduce the workload of doctors and nurses and improve operation efficiency.
Attention:
It is not suitable for the incision of the operation, which is required for docimasia after operation.
It is prohibited to use the disposable skin stapler in suture of interior tissues.
The product is prohibited to use when the package is broken.
The product is prohibited to use when it exceed the valid date.
The product is sterilized and discard for single use, repeat use is prohibited.
The operation doctor should be trained.
Available Certificates:
CE 0197
ISO 13485
Free Sales certificate (CN & EU)
CFDA registration certificate